Metallic Hydrogen & Exotic Covalent Bonding
At extreme pressures, hydrogen becomes metallic — and familiar chemistry gives way to new bonding rules. Recent work shows carbon, oxygen and nitrogen can form “hyper‑hydrocarbons” like CH6 and C2H8, stabilised by the metallic environment. Metallic hydrogen in compounds form room temperature superconductors
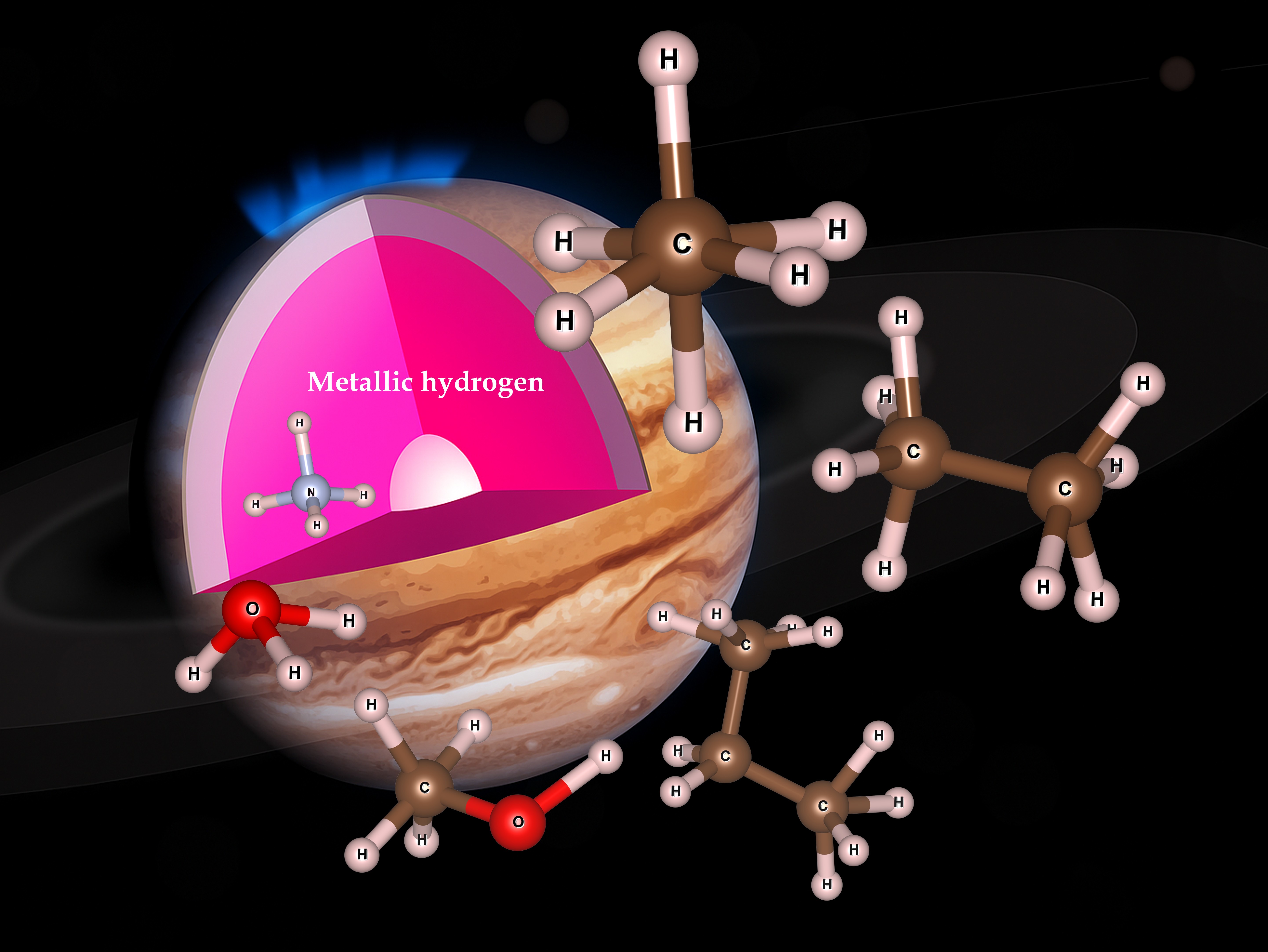
The chemistry is unfamiliar: the heavier atoms borrow electrons from the metallic hydrogen to make extra bonds. It is unknown quite how complicated this new organic chemistry could become.
In fact, almost all elements are soluble in metallic hydrogen (Helium and Neon are not). This explains why spaceprobes cannot find th ecore region of Jupiter and Saturn - it has simply dissolved away.
References
- Seeyangnok, Pinsook & Ackland, Hydrogenation of saturated organic and inorganic molecules in metallic hydrogen, Nature Communications (2025)
- Seeyangnok, Pinsook & Ackland, Solid solubility in metallic hydrogen Phys.Rev.B (2025)
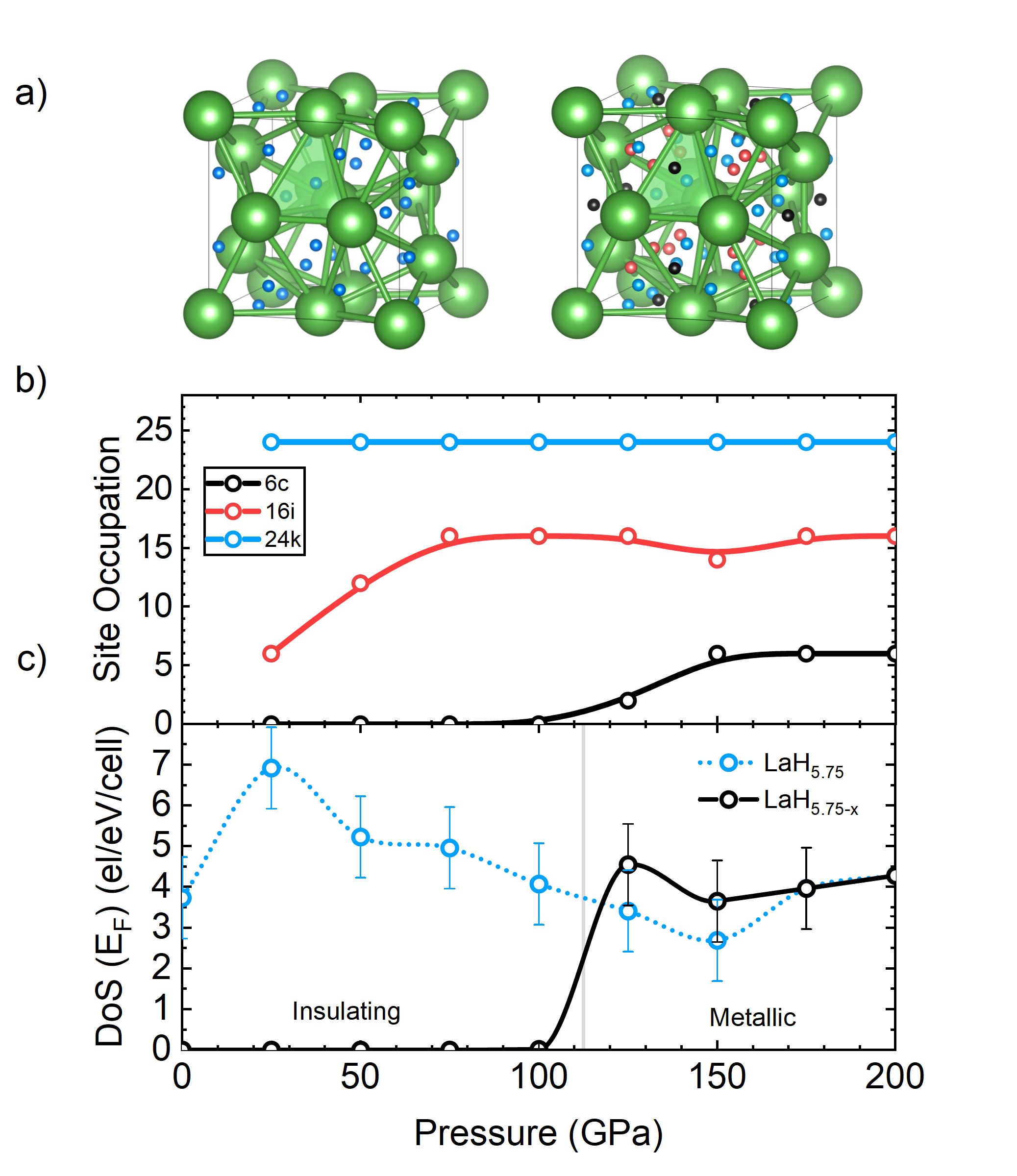
- Seeyangnok, Pinsook & Ackland, Enhanced Electron–Phonon Coupling and Superconductivity in Ba-Alloyed A15 LaH5.75, arXiV (2026)
- M. Peña-Alvarez, G.J. Ackland, and others, Chemically Assisted Precompression of Hydrogen Molecules in Alkaline-Earth Tetrahydrides (2022)
- M.Marques, G.J. Ackland, and others, H2, Chemical Bond in a High-Pressure Crystalline Environment (2023)
- M. Peña-Alvarez, G.J. Ackland, and others, Chemically Assisted Precompression of Hydrogen Molecules in Alkaline-Earth Tetrahydrides
- Ackland & Loveday, Structures of solid hydrogen at 300 K, Phys. Rev. B 101, 094104 (2020)