FRAP on lysozyme diffusion in lipidic cubic phases
contact: Shinpei Tanaka, Stefan Egelhaaf, and Wilson Poon
admin
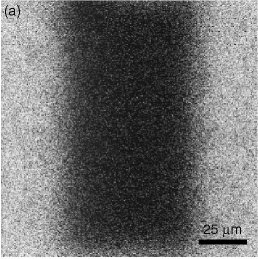
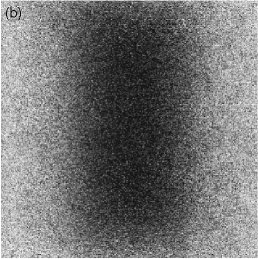

Figures 1(a)-(c) above show a typical FRAP measurement. Only lysozyme molecules are dyed so that we can trace their movement. From the time scale of the fluorescence recovery we could estimate the diffusion coefficient as 4-10 micron^2/s, which is significantly smaller than the one in bulk solutions, 140 micron^2/s. The reason of this slow diffusion is twofold: Firstly, lysozyme molecules are confined in cavities (or `cells') which is a wide part of the water channels, and they have to find narrow paths connecting these cavities in order to diffuse. Secondly, passing through the narrow paths is accompanied by the deformation of bilayers since lysozyme molecules are slightly larger than the path. We are now trying to construct more quantitative model to describe the effect of the confinement on lysozyme diffusion.