Ammonia forms three stochiometric hydrates, a hemihydrate,
(NH3)2.H2O (AHH), a monohydrate, NH3.H2O
(AMH), and a dihydrate, NH3.(H2O)2 (ADH).
These three hydrates are the amongst the simplest systems to have mixed
N-H...O and O-H...N hydrogen bonds — hydrogen bonds of these types are involved
in the base pairings of DNA — and good systems to explore the changes in
geometry with pressure. In addition, the planets Uranus and Neptune contain
large amounts of ammonia and water and the properties of the ammonia hydrates.We
have been using the both the Paris-Edinburgh neutron cell and the Edinburgh
group's facilities for synchrotron x-ray diffraction to study the ammonia
hydrates at both ambient and high pressures.

|
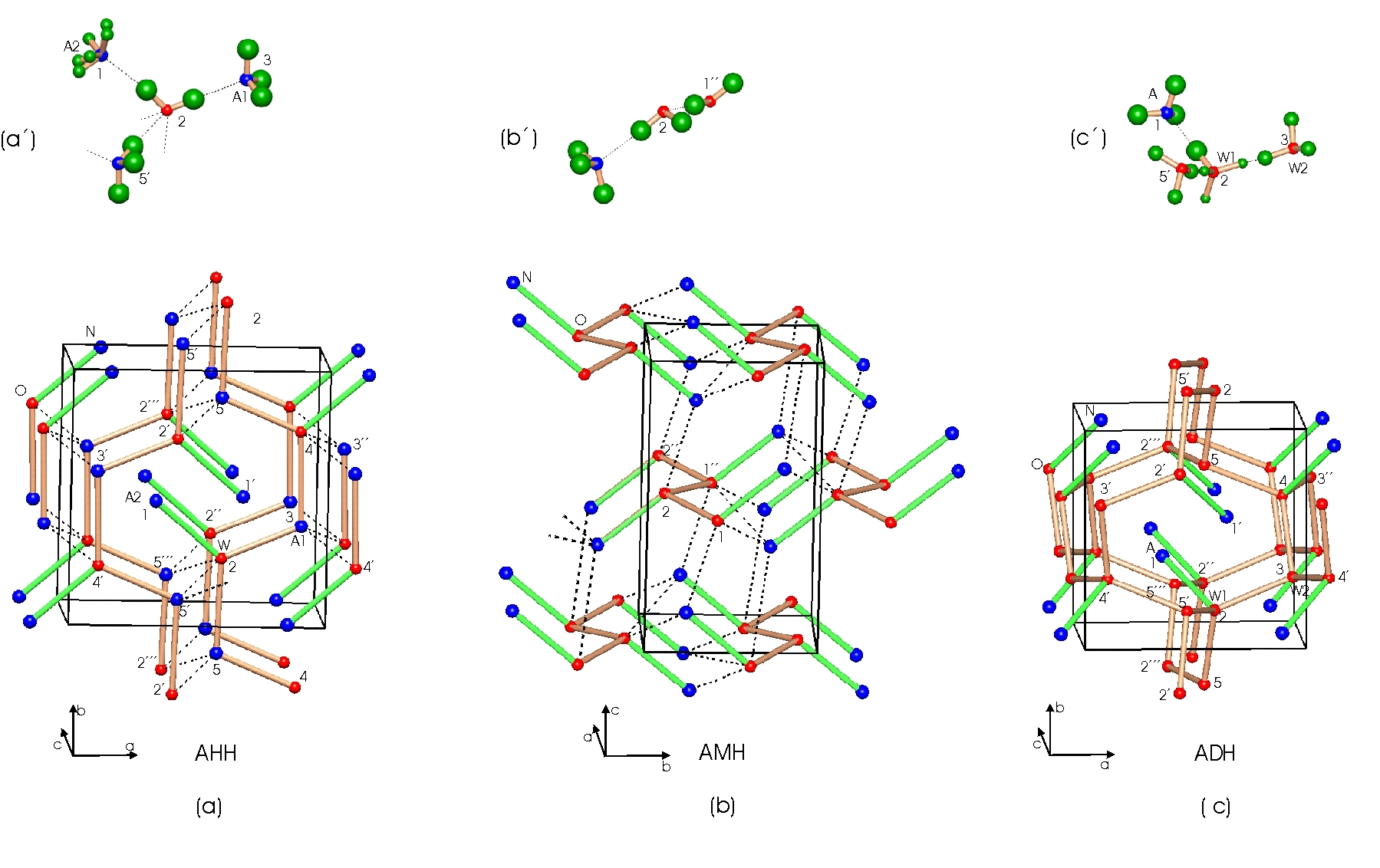
The ambient pressure structures of ammonia hemihydrate (AHH, left) ammonia monohydrate (AMH centre) and ammonia dihydrate (ADH right). The upper diagrams show a part of the H-bond networks including H positions determined from neutron diffraction.
We first had to find the hydrogen positions for the ambient pressure structures
which are shown in the upper parts of the diagram above. The H-bond networks
of AHH and ADH are quite similar and have form channels in which sit ammonia
molecules. The blue circles are the nitrogen atoms of the ammonia molecules
and the red circles are the oxygen atoms of the water. AMH has a 3-d network
of bonds without obvious channels.
So far we have explored AMH in detail under pressure. It has at least six high-pressure phases and a complex phase diagram in that, while all the phase transitions which occur on compression can be reversed by decompression, none of the changes which occur on warming are reversed by cooling.
We have solved the structure of AMH-VI. It has a body centred cubic arrangement
of molecular centres with substitutional disorder of ammonia and water molecules
so each molecular centre has a 50% change of being occupied by
either a water or ammonia molecule. AMH-VI is thus a new kind of material.
— A hydrogen bonded molecular alloy.