Miguel Martinez-Canales
Research
These are some highlights, in no particular order, of the research I've performed so far. A complete list of my publications can be found here.
Hydrogen
|
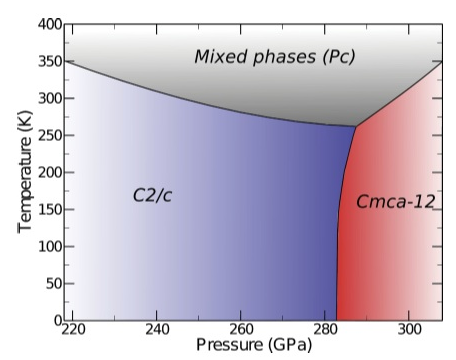
Hydrogen is the hottest topic in high pressure physics. An isolated atom is one of the few exactly resoluble problems in quantum mechanics. The H2+ molecule is a typical example in the classrom. But at high pressure, hydrogen stops behaving the way we are used to. We believe metallic hydrogen is the main cause of Jupiter's strong magnetic field, for example, and predictions hint metallic H will be superconducting with a high Tc There has been a strong experimental push in the last few years to metallize hydrogen. This is a hard problem and, furthermore, getting experimental information is also difficult. The left figure depicts a theoretical P-T phase diagram, which suggests hydrogen adopts a mixed atomic and molecular structure, explaining the semiconducting behaviour seen experimentally. |
Hydrides under pressure
|
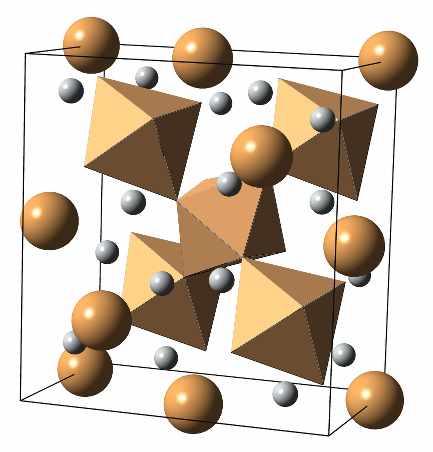
Metallization of Hydrogen is hard and, at room temperature, it happens well above 300 GPa (3 million atomospheres). Neil Ashcroft suggested in 2004 that hydrogen-rich compounds would preserve most of the properties of metallic H, while lowering the metallization pressure. This effect is called "chemical precompression". Ashcroft went one step further, and suggested that hydrogen-righ alloys should also have a high superconducting Tc, like hydrogen. Most of my PhD thesis was spent figuring out whether SiH4 and other Group IV hydrides behaved according to this idea. There were initial reports that silane was a reasonable BCS superconductor, but calculations first, and then further experiments, reinterpreted the original experiment. According to our calculations, silane polimerizes at around 50 GPa, protecting its electronic gap. Eventually, it is seen to superconduct, but at very low temperatures, and high pressures. The figure on the left displays the onset of polimerization of silane, at 50 GPa |
Terapascal physics
|
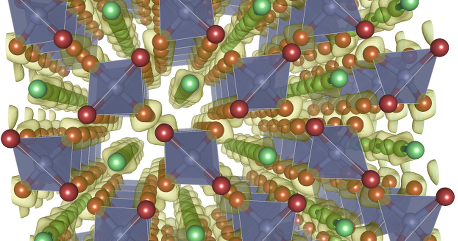
At ambient pressure, bonding and simple cheminstry are well understood. Simplifying substantially, atomic orbitals hybridize following familiar energetic rules and electrons fill the resulting levels with well known geometries. However, at very high pressures, the familiar rules break apart. The reason for that is an increased orbital overlap. Furthermore, electrons can't really get closer to the nuclei: the inner electrons are very stable, so Pauli's exclusion principle keeps valence states far from the core. This effect is responsible of the Metal-Insulator transition in Li and Na. Structure at high pressure can change so much that CO2 turns into a material similar to silica, and nitrogen breaks its strong triple bond to form connected covalent network. The advent of shock facilities, reaching tens to hundreds of million atmospheres brings the need to understand chemistry and structure at conditions far beyond what is easy to experiment with. Part of my research consists of building up models for structures and chemistry based on computer simulations The picture on the left depicts a metallic salt structure that nitrogen is believed to adopt in the TPa region. All spheres are N atoms, and the different colours represent the different charge on them. |
Structure search
Borane
|
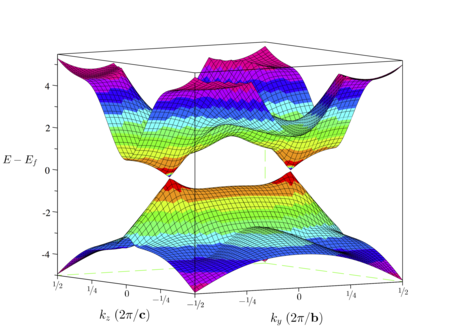
Boron is an exciting material. Many alotropes, similar bonding to carbon. Long quest for monolayer There's a rich boron hydride chemistry, mostly based on broken icosahedra. We predict the existence of a relatively stable BH monolayer, that may be stabilised through high-pressure synthesis. This monolayer is predicted to have a Dirac cone, as in graphene. Unlike graphene, due to broken symmetry in the boron sites, the Dirac cone in 2D borane is resilient due to electron conservation sum rules. |
