Equipment regularly used by group members
Most of the equipment in this list is not owned by the group, but is available to us in the School of Physics & Astronomy at The University of Edinburgh.
Confocal microscopy
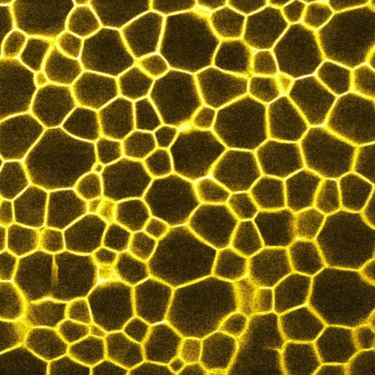
Confocal microscopy can be used to characterize the 3D microstructure of (typically) fluorescently labelled samples in-situ and in real time at an optimal resolution of 150 nm lateral and 500 nm axial. As it uses laser light to excite the fluorescent dye and then records the emission light, it works best on samples that can be refractive-index matched i.e. those in which all components of the sample have the same refractive index. All the same, useful information can also be obtained from opaque samples, e.g. about the microscopic structure at the edge of the sample.
We usually employ a Zeiss LSM700 confocal microscope, but we also have access to a Leica TCS SP8 tandem scanner for imaging at 25 fps.
Cryo FIB-SEM
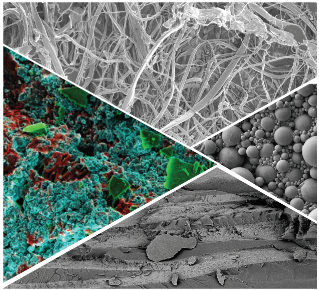
Our Zeiss Crossbeam 550 enables us to image beam sensitve samples at extremely high resolution, 1.4 nm at beam energies as low as 1 kV, so that we can visualise the internal structure of formulations, composites, biological matter and other materials. Charge compensation enables the use of uncoated samples, whilst advanced tools such as automatic image acquisition and stitching can be used to achieve high-resolution images over large areas. Multiple detectors (In-lens, Secondary Electron and Backscattered Electron) give different information about the sample simultaneously.
The FIB column enables the controlled milling away of thin slices of the sample. Between each slice an SEM image is taken automatically, thus generating a stack of images that we can reconstruct to obtain the 3D volume of the sample.
An Oxford Instruments Xmax 150 EDS system and a backscatter detector gives rapid and easy elemental contrast, allowing determination of the chemistry of the sample and therefore enabling identification of phases and spatial distributions.
With our Quorum Technologies PP3010T cryo preparation system, we can slush freeze, freeze-fracture, sublime and sputter coat samples before transferring to the main SEM chamber, all under cryo conditions; we also have a high-pressure freezer. We can combine all the above techniques to study frozen liquids, emulsions and biological samples in close to their natural state.
For more information and to use the instrument please visit the instrument website or contact the instrument scientist.
Digital photography
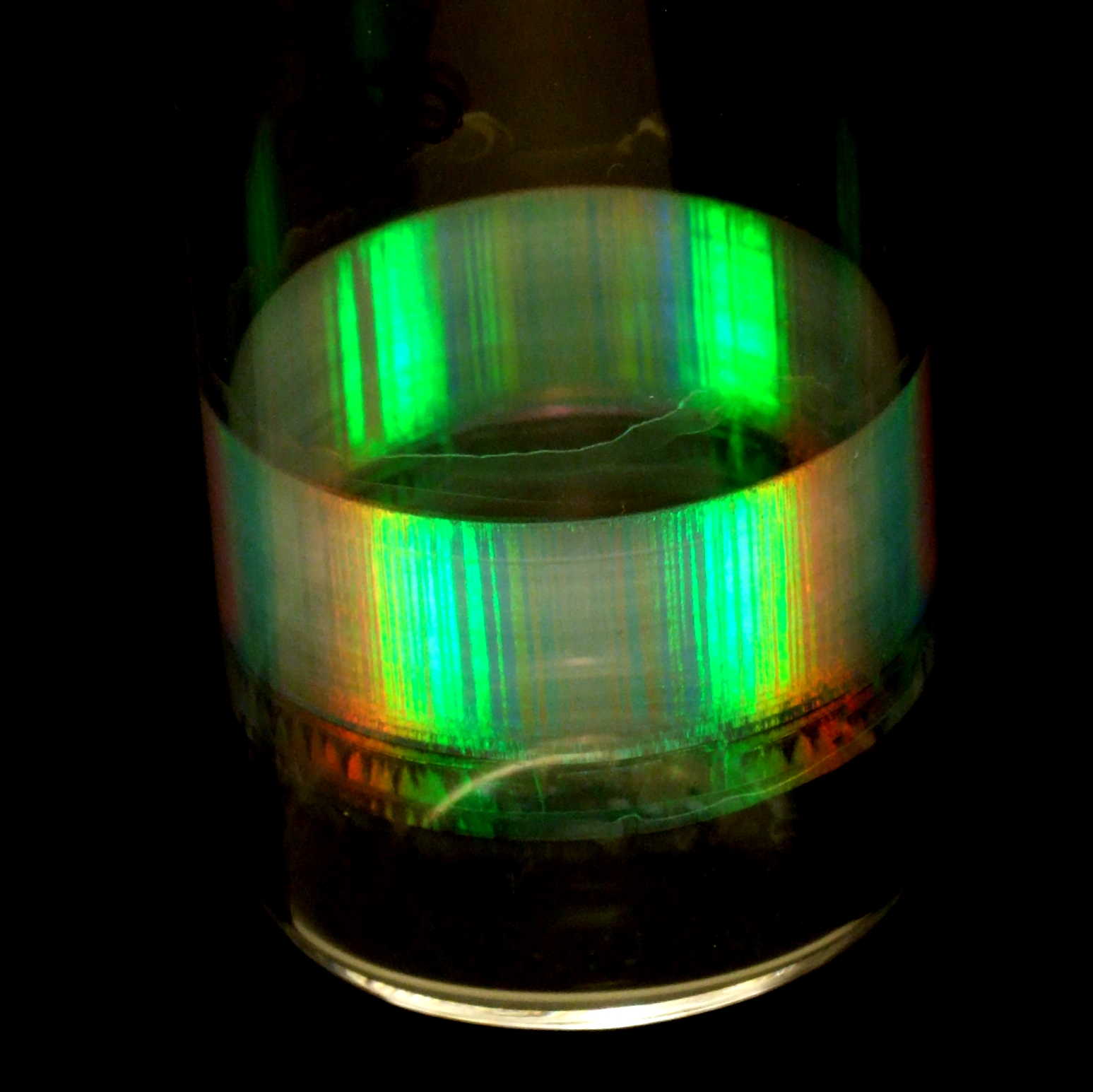
Digital photograph, or simply visual observation, can be very useful when characterizing soft materials, as it often reveals useful information about the sample quickly. In this photograph, for example, the Bragg colours under white-light illumination suggest that the sample consists of sub-micron spheres arranged in a crystalline packing; the dark stripes suggest crystal twinning.
For visual observations, we use a range of equipment including Raspberry Pi operated webcams and digital SLR cameras.
Potentiostat / galvanostat
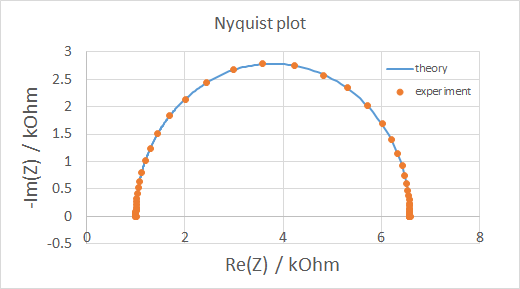
A potentiostat / galvanostat is used for electrochemical charcaterization of samples. Simply speaking, either the voltage across (potentiostat) or the current through (galvanostat) the sample is controlled, while the current/voltage is measured. We mostly use this machine for cyclic voltammetry (essentially generating I-V curves) and electrochemical impedance spectroscopy (plotting imaginary vs real part of impedance).
We usually employ a VersaSTAT 3 from Princeton Applied Research, supplied by Blue Scientific (UK).
Scanning Electron Microscopy (SEM)

In Scanning Electron Microscopy (SEM), electrons rather than light are used for imaging samples. This allows for higher resolution, but only the sample surface can be imaged and typically the sample has to be imaged under vacuum i.e. not in solution. The SEM we have access to can image secondary electrons (for a topographical view as in the image) or backscattered electrons (for a compositional view).
We usually employ a JEOL JSM-6010, which has a low-vacuum mode and can be equipped with a cool stage; we also have access to a Transmission Electron Microscope (TEM) via the School of Biological Sciences.
Static light scattering
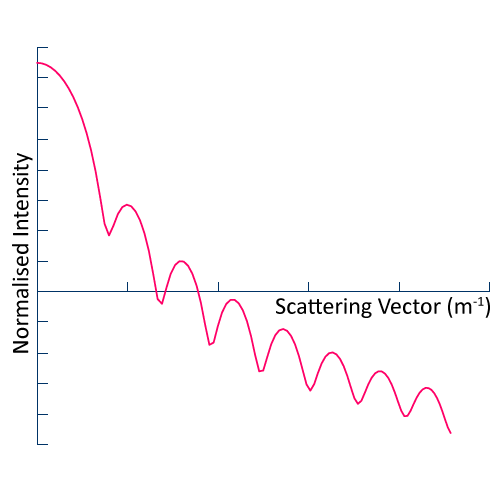
In Static Light Scattering (SLS), we measure the scattered intensity as a function of scattering angle for a suspension of colloidal particles i.e. liquid or solid particles suspended in a liquid. By fitting the data to an appropriate model for Mie scattering, we obtain the average particle size and variance therein (particle size range 100 to 2000 nm). SLS can also be used to determine molecular weight.
We usually employ an ALV LSE-5004 based set-up, which allows for temperature controlled measurements in which the scattering angle can be varied between 20 and 150 degrees.
X-ray micro-CT
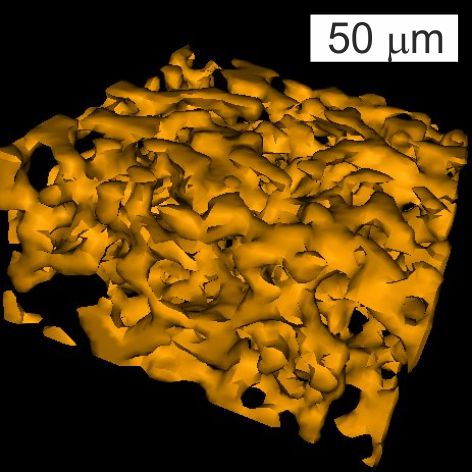
In X-ray micro-CT (CT), we essentially take X-ray transmission images of a sample at various angles covering a full 360 degrees rotation. The resulting images are then analyzed by a computer software program that can reconstruct them into a 3D image stack: a series of 2D images at various heights throughout the sample. One advantage of X-rays is that most materials are relatively transparent to X-rays, so full 3D constructions of visually opaque materials can be made (see image).
We usually employ a Bruker Skyscan 1172 desktop system at the Department of Orthopaedics & Trauma at The University of Edinburgh (Royal Infirmary).